Ultrafast Optics in Microscopy: Recent Advances
Section n. 1
3D Imaging with Femtosecond Pulses
Combining 3D Imaging with Ultrafast Optics
Written by Martin Hörmann (ESR4)
A recent collaboration between ICFO (Barcelona) and Politecnico di Milano focused on combining the previously rather separated realms of 3D imaging and ultrafast optics. Namely, the 3D imaging technique of Optical Diffraction Tomography (ODT), which is well established in the bio and cell-imaging area, has been demonstrated with light pulses having a pulse duration as low as twenty femtoseconds and in turn a spectrum of more than 100nm bandwidth. There is potential impact in both directions: 3D (live) cell-imaging can profit from ultrafast pump probe techniques whereas ODT can be applied to study also ultrafast processes in 3D in material science, such as photoinduced charge-carrier diffusion in semiconductors.
Tomography retrieves the three-dimensional structure of an object. In many cases images (2D projections) of the object can be acquired, such as x-ray images of bones in the human body, and the missing third dimension can be resolved by acquiring images from all around 180° of the sample by use of the Radon Transform. This is called Projection Tomography and is utilized in Computed Tomography (CT scan) with x-rays in hospitals. ODT is the follow-up of this technique concerning also diffraction, as occurs by cell imaging where the size of the object and the wavelengths used are of similar order. The connection between images and 3D structure is established in a more complicated manner and requires amplitude and phase of the electric field, in contrast to intensity only [1], [2]. Further, rotating the source and detection part becomes a major problem with cells in microscopes. Therefore, the standard ODT setup up to now consists of an interferometric setup in which the detection part and sample are fixed but the angle of the incoming beam is scanned with a condenser lens. Then, to retrieve amplitude and phase, the image field interferes with a reference wave on the camera (off-axis holography). The holography constrains the light sources to have a long coherence length, in order to keep the interference for different illumination angles. This is the exact opposite property of femtosecond pulses, which are the shortest existing light pulses in the visible. Furthermore, narrowband light sources introduce severe speckle noise.
In order to remove this speckle noise and to move to more easy setups there popped up many creative ways in the recent years in the optical imaging field. Some approaches keep the holographic scheme and elaborately compensate for broadband illumination [3], [4] or use shear interferometry [5]. Other approaches only record intensity images mainly with LEDs as light source and either recover the complex field prior to tomography [6], as Transport of Intensity Equation [7] or only use the measured intensity fields [8]. These methods rely solely on nonlinear inverse problem solver which play an increasingly bigger role in this field.
The current work carried out by one ESR in in the group of Niek van Hulst in ICFO, Barcelona, concentrated on the “hardware” side. The aim was to ultimately use broadband light sources to do ODT in a classical holographic configuration. To start, an easy microscopic system with low resolution was built to understand the basics of ODT and 3D reconstruction. Then, step by step, the complexity of the setup is increased by going to more broadband light sources. With decreasing the pulse length, the challenge consists of keeping interference of the two pulses which were in the end only a few tens of micrometers long over the entire camera chip for different illumination angles. It was shown that in order to fully interfere 20 femtosecond pulses from a Titanium Sapphire oscillator with a bandwidth of more than 100nm, the pulses of the two interferometric branches need to have exactly the same properties as the chirp introduced by dispersive optical elements, propagation distance as well as position and curvature on the camera chip. Towards the end, proof-of-principle experiments were conducted showing the reconstruction of objects at the full bandwidth of the Ti:Sapphire oscillator in a hyperspectral approach allowing to keep the short pulse durations when acquiring the spectrum. Therefore this system can be straight-forwardly implemented in already existing widefield pump probe schemes [9] to combine ultrafast optics with 3D imaging.
Section n. 2
From 3D to Polarisation to Single Nanoparticle Microscopy
Probing Biological Molecular Organization with Polarized Light Microscopy
Written by Eleanor Beatrice Munger (ESR6)
Optical microscopy helps visualize biological subjects at the smallest scale without disrupting the sample itself. But the very size of light means that it cannot interrogate a space smaller than 100 nm, this limit is known as the diffraction limit. The molecules making up cells and tissues are 0.1-10 nm in length, and thus hundreds to thousands of molecules are seen in each pixel of light in a microscope image (when you take into account volume of the pixel). Current advances in biomedical optical imaging challenge the diffraction limit in a variety of methods in order to improve biological understanding with higher spatial resolution in order to approach the molecular scale. To this end, imaging the molecular organization with a diffraction-limited spot can provide much information about the properties of biological material that can be exploited for biomedical understanding. In order to probe sub-micron molecular organization, polarized light can interact with biological matter to reveal the average orientation and distribution of molecules within a focal volume.
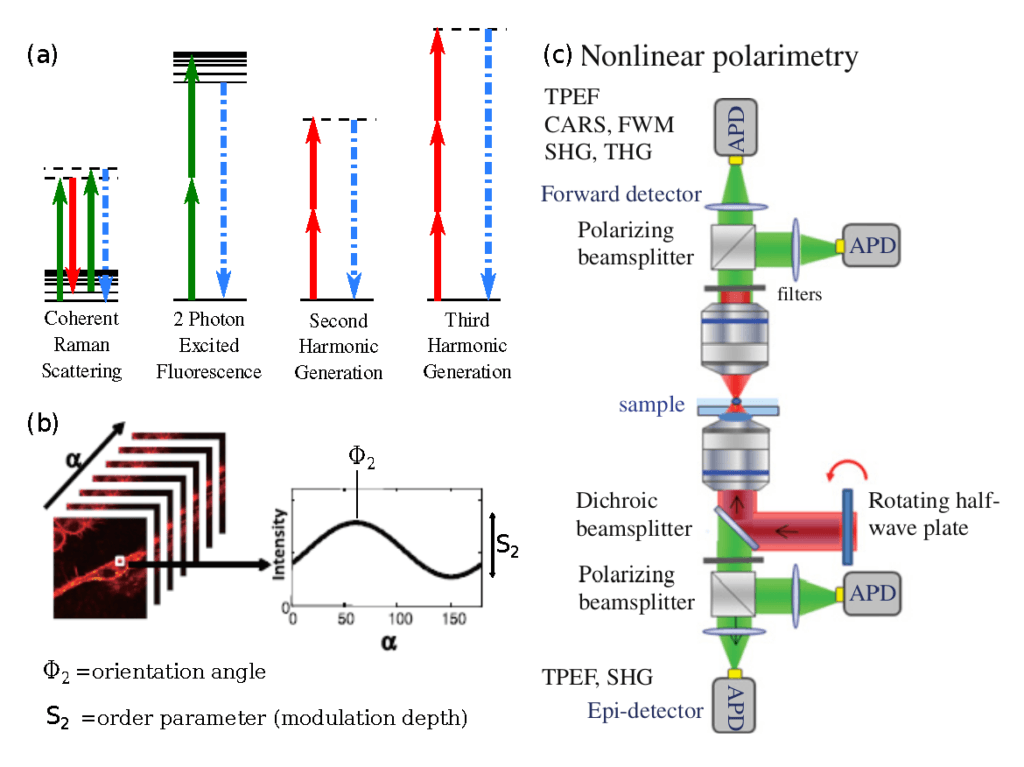
In order to draw response from molecules within such a small sample volume, nonlinear spectroscopy, such as coherent Raman scattering (CRS) or second/third harmonic generation (SHG/THG), is used to generate sufficient signal for efficient data collection.
Coherent Raman scattering is a chemically selective technique which can probe the molecular orientation of C-H bonds within lipid structures in biological material. Since Raman scattering probes the polarizability of bond vibrations, matching the excitation polarization to the bond’s orientation increases the intensity of the emitted signal. Thus, by sequentially measuring the intensity change while modulating the excitation polarization, the orientation and molecular order of the selected bonds can be probed. Polar-CRS imaging has been employed to measure the molecular organization of single lipid membranes, but it is more fruitful in larger lipid structures such as myelin sheaths in neuro-degenerative diseases such as Krabbe Disease [10] and Multiple Sclerosis [11] [12]. Furthermore, the speed of polarized CRS measurements is improving, with molecular organization images taken in 0.25-1s to allow for minute timescale processes to be monitored in biological systems [13].
Other nonlinear contrasts provide material-specific polarized measurements, as the selection rules for Sec- ond Harmonic Generation and Third Harmonic Generation rely on the centrosymmetry and anisotropy of a homogenous material. Polarized SHG/THG measurements probe different molecular polarizabilities than CRS, providing complementary molecular organization information. Collagen birefringence makes it especially sus- ceptible to SHG imaging, this has been capitalized upon in pr-SHG medical imaging [14] [15]. Ducorthial et al demonstrate the new technological advances of fast pr-SHG (0.25-0.5s images) to measure increase in molecular organization of collagen in murine skin as it is stretched. Similarly, pr-THG can probe organization of crystalline or isotropic structures within biological samples, such as lipid phase changes in lipid droplets [16] and crystalline particles in zebrafish embryos [17].
Polarization control has aided fluorescence microscopy as well, with the novel super-resolution by polarization demodulation (SPoD) microscopy capitalizing on fluorescence anisotropy to resolve fluorophore position below the diffraction limit [18] [19] [20]. Without photo-switching fluorophores as in STORM/PALM super-resolution imaging, the modulated response of distinctly arranged fluorophores in different positions within the point-spread- function to a modulating polarization allows for their localization within 50nm [18] [19] [20].
In conclusion, polarization-based nonlinear optical microscopy provides a molecular-orientation based readout for biological morphology. Such molecular orientation information also helps improve the imaging capability of super-resolution fluorescence microscopy improving the resolution of biomedical imaging.
Section n. 3
Spectroscopy in wide field imaging
Imaging for spectroscopy
Written by Saurabh Ishwar Borkar (ESR9)
Spectrosocopy is the study of material interaction with light at different wavelengths: – its experimental challenges include resolving power, light collection efficiency, wavelength accuracy, and signal-to-noise ratio. A typical spectrograph is recorded by passing a beam of white light through a sample of the material and the white light is then dispersed. The dispersed light, the spectrum is analyzed either by moving a narrow slit across it, recording what passes through the slit with a suitable detector point detector, or by direct recording on a multiplex camera. If the slit is narrow in order to see fine details, then very few photons reach the detector and the result is a low signal-to-noise ratio. All spectrographs operate using fundamental geometric optics. Light strikes wavelength dispersive element at a certain angle of incidence and depending on the type of dispersive material such as grating or prism, the light is either diffracted or refracted. Thus, multiple wavelengths are recorded to characterize a sample.
Several applications in biology and chemistry involve the study of (metallic) nanoparticles and their interactions with molecules [22] and cells [23], and many of the fundamental properties of nanoparticles are under study. In recent years, advances in imaging and spectroscopy allow us to look at single nanoparticles [24] and record their spectral response [25]. A typical experimental approach divides the scattered light into an imaging arm, which records the dark field image of nanoparticles, and a spectroscopy arm, which collects the light from a selected individual nanoparticle, often using a fiber connected to spectrometer. This split detection approach has two disadvantages: First, a reduced number of photons detected in the spectrometer, as half the scattered light is used for imaging. Second, this approach is very time-consuming since only single point spectra from one particle is recorded at any given time.
One can overcome these limitations by imaging the complete field of view (wide field configuration) to obtain the spectrum of individual particles all at once. To extract the spectral information, one relies on Fourier transform spectroscopy (FTS) [26]. FTS is based on a conventional Michelson interferometer [27] where a broadband source (SuperK) is split into two arms each having mirrors shown in figure 2A. The interference between the lights reflected off stationary and moving mirrors generate what is called an interferogram, a modulated signal as a function of displacement of a moving mirror. This signal encodes the wavenumber information of the source/sample. A Fourier transform performed on the interferograms gives the desired spectrum (Figure 2B).
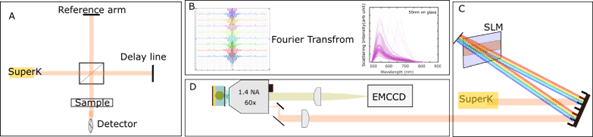
A more practical and phase-stable way to implement Michelson interferometer is using a spatial light modulator (SLM), as shown in figure 1C which generates time delay-dependent interferograms shown in figure 2B. The EMCCD then records multiple time delay-dependent images of the sample. This image set contains the spectral interferograms. A Fourier Transform of these interferograms gives the desired spectra at each image pixel. Figure 2B shows the scattering from 50nm spherical gold nanoparticles. This technique of FTS is not just limited to nanoparticles but can also be used to perform single molecule non-linear ultrafast spectroscopy relying on fluorescence [28]. In principle the concept can be extended to other wavelength ranges such as mid-infrared where the fundamental vibrational modes of molecules exist yet due to the lack of availability of photodetector arrays in mid-infrared, this technique is so far limited to a single photodetector. The technique is fast compared to point scanning single nanoparticles. With just recorded images at different time delays, one can retrieve spectral information of hundreds of individual nanoparticles. This technique of FTS eliminates the need of having a spectrometer and can be implemented in a widefield configuration (shown in figure 2D) to image hundreds of nanoparticles at once.
About the Authors
The MUSIQ Innovation Newletter n. 5 was written by three of the Early Stage Researchers (ESRs) working on individual projects as part of the MUSIQ project.

Martin Hörmann (ESR4)
Martin is hosted at the Physics department at the Politecnico di Milano in Italy. He is working on 2D Electronic Spectroscopy of artificial light-harvesting systems.

Eleanor Munger (ESR6)
Eleanor is hosted at the Centre National de la Recherche Scientifique, Institute Fresnel, France. Her research project focuses on Imaging molecular organisation in myelin sheaths through high speed polarisation controlled nonlinear microscopy.
Saurabh Ishwar Borkar (ESR9)
Saurabh is hosted at the Institute of Photonic Sciences, Molecular Nanophotonics group in Spain. His research focuses on coherent ultrafast electronic and vibrational molecular dynamics of single molecules at room temperature using fluorescence readouts.
References
[1] E. Wolf, “Three-dimensional structure determination of semi-transparent objects from holographic data,” Opt. Commun., vol. 1, no. 4, pp. 153–156, 1969, doi: 10.1016/0030-4018(69)90052-2.
[2] Y. Sung, W. Choi, C. Fang-Yen, K. Badizadegan, R. R. Dasari, and M. S. Feld, “Optical diffraction tomography for high resolution live cell imaging,” Opt. Express, vol. 17, no. 1, p. 266, Jan. 2009, doi: 10.1364/OE.17.000266.
[3] C. Park, K. Lee, Y. Baek, and Y. Park, “Low-coherence optical diffraction tomography using a ferroelectric liquid crystal spatial light modulator,” Opt. Express, vol. 28, no. 26, p. 39649, 2020, doi: 10.1364/oe.405418.
[4] K. R. Lee, S. Shin, Z. Yaqoob, P. T. C. So, and Y. K. Park, “Low-coherent optical diffraction tomography by angle-scanning illumination,” J. Biophotonics, 2019, doi: 10.1002/jbio.201800289.
[5] U. Nska, “Common-path intrinsically achromatic optical diffraction tomography,” vol. 12, no. 7, pp. 4219–4234, 2021.
[6] A. B. Ayoub, A. Roy, and D. Psaltis, “Optical Diffraction Tomography Using Nearly In-Line Holography with a Broadband LED Source,” Appl. Sci., vol. 12, no. 3, 2022, doi: 10.3390/app12030951.
[7] J. Li, Q. Chen, J. Zhang, Z. Zhang, Y. Zhang, and C. Zuo, “Optical diffraction tomography microscopy with transport of intensity equation using a light-emitting diode array,” Opt. Lasers Eng., vol. 95, no. January, pp. 26–34, 2017, doi: 10.1016/j.optlaseng.2017.03.010.
[8] S. Chowdhury et al., “High-resolution 3D refractive index microscopy of multiple-scattering samples from intensity images,” Optica, vol. 6, no. 9, p. 1211, 2019, doi: 10.1364/optica.6.001211.
[9] M. Liebel, F. V. A. Camargo, G. Cerullo, and N. F. van Hulst, “Ultrafast transient holographic microscopy,” Nano Lett., vol. 21, no. 4, pp. 1666–1671, 2021, doi: 10.1021/acs.nanolett.0c04416.
[10] G. de Vito, V. Cappello, I. Tonazzini, M. Cecchini, and V. Piazza, “RP-CARS reveals molecular spatial order anomalies in myelin of an animal model of Krabbe disease,” eng, Journal of Biophotonics, vol. 10, no. 3, pp. 385–393, Mar. 2017, issn: 1864-0648. doi: 10.1002/jbio.201500305.
[11] P. Gasecka, A. Jaouen, F.-Z. Bioud, H. B. d. Aguiar, J. Duboisset, P. Ferrand, H. Rigneault, N. K. Balla, F. Debarbieux, and S. Brasselet, “Lipid order degradation in autoimmune demyelination probed by polar- ization resolved coherent Raman microscopy,” en, bioRxiv, p. 105 965, Feb. 2017, Publisher: Cold Spring Harbor Laboratory Section: New Results. doi: 10 . 1101 / 105965. [Online]. Available: https :/ / www . biorxiv.org/content/10.1101/105965v1 (visited on 05/12/2020).
[12] G. de Vito, I. Tonazzini, M. Cecchini, and V. Piazza, “RP-CARS: Label-free optical readout of the myelin intrinsic healthiness,” EN, Optics Express, vol. 22, no. 11, pp. 13 733–13 743, Jun. 2014, Publisher: Optical Society of America, issn: 1094-4087. doi: 10.1364/OE.22.013733. [Online]. Available: https://www. osapublishing.org/oe/abstract.cfm?uri=oe-22-11-13733 (visited on 05/12/2020).
[13] M. Hofer, N. K. Balla, and S. Brasselet, “High-speed polarization-resolved coherent Raman scattering imaging,” EN, Optica, vol. 4, no. 7, pp. 795–801, Jul. 2017, Publisher: Optical Society of America, issn: 2334-2536. doi: 10 .1364 / OPTICA.4 .000795. [Online]. Available: https:// www.osapublishing.org/ optica/abstract.cfm?uri=optica-4-7-795 (visited on 04/21/2020).
[14] K. R. Campbell, R. Chaudhary, J. Handel, M. Patankar, and P. J. Campagnola, “Polarization Resolved Second Harmonic Generation Imaging of Human Ovarian Cancer Tissues Reveals Macro/Supra Molecular Changes in Collagen Structure,” in Optics in the Life Sciences Congress, ser. OSA Technical Digest (online), Journal Abbreviation: NTM, San Diego, California: Optical Society of America, Apr. 2017, NW3C.3. doi: 10.1364/NTM.2017.NW3C.3. [Online]. Available: http://www.osapublishing.org/abstract.cfm?URI= NTM-2017-NW3C.3.
[15] G. Ducourthial, J.-S. Affagard, M. Schmeltz, X. Solinas, M. Lopez-Poncelas, C. Bonod-Bidaud, R. Rubio- Amador, F. Ruggiero, J.-M. Allain, E. Beaurepaire, and M.-C. Schanne-Klein, “Monitoring dynamic col- lagen reorganization during skin stretching with fast polarization-resolved second harmonic generation imaging,” Journal of Biophotonics, vol. 12, no. 5, e201800336, May 2019, Publisher: John Wiley & Sons, Ltd, issn: 1864-063X. doi: 10 . 1002 / jbio. 201800336. [Online]. Available: https:// onlinelibrary. wiley.com/doi/full/10.1002/jbio.201800336 (visited on 04/03/2020).
[16] G. Bautista, S. G. Pfisterer, M. J. Huttunen, S. Ranjan, K. Kanerva, E. Ikonen, and M. Kauranen, “Polar- ized THG Microscopy Identifies Compositionally Different Lipid Droplets in Mammalian Cells,” English, Biophysical Journal, vol. 107, no. 10, pp. 2230–2236, Nov. 2014, Place: Cambridge Publisher: Cell Press WOS:000345195700004, issn: 0006-3495. doi: 10.1016/j.bpj.2014.10.009.
[17] J. Morizet, G. Ducourthial, W. Supatto, A. Boutillon, R. Legouis, M.-C. Schanne-Klein, C. Stringari, and E. Beaurepaire, “High-speed polarization-resolved third-harmonic microscopy,” EN, Optica, vol. 6, no. 3, pp. 385–388, Mar. 2019, Publisher: Optical Society of America, issn: 2334-2536. doi: 10.1364/OPTICA.6. 000385. [Online]. Available: https://www.osapublishing.org/optica/abstract.cfm?uri=optica-6- 3-385 (visited on 04/03/2020).
[18] N. Hafi, M. Grunwald, L. S. van den Heuvel, T. Aspelmeier, J.-H. Chen, M. Zagrebelsky, O. M. Schuette, C. Steinem, M. Korte, A. Munk, and P. J. Walla, “Fluorescence nanoscopy by polarization modulation and po- larization angle narrowing,” English, Nature Methods, vol. 13, no. 1, pp. 101–101, Jan. 2016, Place: London Publisher: Nature Publishing Group WOS:000367463600035, issn: 1548-7091. doi: 10.1038/nmeth0116- 101a.
[19] K. Zhanghao, L. Chen, X.-S. Yang, M.-Y. Wang, Z.-L. Jing, H.-B. Han, M. Q. Zhang, D. Jin, J.-T. Gao, and P. Xi, “Super-resolution dipole orientation mapping via polarization demodulation,” English, Light-Science & Applications, vol. 5, e16166, Oct. 2016, Place: Changchun Publisher: Chinese Acad Sciences, Changchun Inst Optics Fine Mechanics and Physics WOS:000387461800002, issn: 2047-7538. doi: 10.1038/lsa.2016. 166.
[20] A. Albrecht, D. Pfennig, J. Nowak, R. Matis, M. Schaks, N. Hafi, K. Rottner, and P. J. Walla, “Amplitude Analysis of Polarization Modulation Data and 3D-Polarization Demodulation (3D-SPoD),” en, bioRxiv, p. 2020.03.10.986034, Mar. 2020, Publisher: Cold Spring Harbor Laboratory Section: New Results. doi: 10.1101/2020.03.10.986034. [Online]. Available: https://www.biorxiv.org/content/10.1101/2020. 03.10.986034v1 (visited on 04/21/2020).
[21] S. Brasselet, “Polarization-resolved nonlinear microscopy: Application to structural molecular and biological imaging,” EN, Advances in Optics and Photonics, vol. 3, no. 3, p. 205, Sep. 2011, Publisher: Optical Society of America, issn: 1943-8206. doi: 10.1364/AOP.3.000205. [Online]. Available: https://www. osapublishing.org/aop/abstract.cfm?uri=aop-3-3-205 (visited on 04/21/2020).
[22] S. Khatua and M. Orrit, “Probing, Sensing, and Fluorescence Enhancement with Single Gold Nanorods,” J. Phys. Chem. Lett., vol. 5, no. 17, pp. 3000–3006, 2014, doi: 10.1021/jz501253j.
[23] J. Kim et al., “Use of Nanoparticle Contrast Agents for Cell Tracking with Computed Tomography,” Bioconjug. Chem., vol. 28, no. 6, pp. 1581–1597, 2017, doi: 10.1021/acs.bioconjchem.7b00194.
[24] J. Ortega-Arroyo and P. Kukura, “Interferometric scattering microscopy (iSCAT): New frontiers in ultrafast and ultrasensitive optical microscopy,” Phys. Chem. Chem. Phys., vol. 14, no. 45, pp. 15625–15636, 2012, doi: 10.1039/c2cp41013c.
[25] M. Liebel, J. T. Hugall, and N. F. Van Hulst, “Ultrasensitive Label-Free Nanosensing and High-Speed Tracking of Single Proteins,” Nano Lett., vol. 17, no. 2, pp. 1277–1281, 2017, doi: 10.1021/acs.nanolett.6b05040.
[26] E. Gellings, R. J. Cogdell, and N. F. Van Hulst, “Room-Temperature Excitation-Emission Spectra of Single LH2 Complexes Show Remarkably Little Variation,” J. Phys. Chem. Lett., vol. 11, no. 7, pp. 2430–2435, 2020, doi: 10.1021/acs.jpclett.0c00375.
[27] A. A. Michelson and E. W. Morley, “On the relative motion of the Earth and the luminiferous ether,” Am. J. Sci., vol. s3-34, no. 203, pp. 333–345, 1887, doi: 10.2475/ajs.s3-34.203.333.
[28] M. Liebel, C. Toninelli, and N. F. Van Hulst, “Room-temperature ultrafast nonlinear spectroscopy of a single molecule,”Nat. Photonics, vol. 12, no. 1, pp. 45–49, 2018, doi: 10.1038/s41566-017-0056-5.

